Chapters
We know that atom is a fundamental particle that accumulates to make up a matter. Today, what we know about atoms is the hard work of the previous scientists who tried hard to figure them out about it. Some of them failed to understand the atom while some of them succeed and their theories are still used today to describe an atom. However, the theories of scientists, who failed to discover the realities of atoms, were not gone to waste. Their theories helped the other scientists to discover the atom and that is why understanding their faulty theories is also an important part of revealing the secrets of an atom. Allow us to take you on a journey of how atoms were discovered?

Dalton's Atomic Model
Nobody knew about atoms until a scientist, named "John Dalton", showed up and proposed a theory. He proposed that every matter consists of tiny balls that are indivisible particles. These particles are called atoms. He described atoms as solid, massy, hard, impenetrable, and moving particles.
Sir John Dalton came up with this law using two other laws. One is the law of mass conservation and the second is the law of constant composition. The law of mass conservation says that the matter in this whole universe is constant. Matter can never be created nor be destroyed but it can change its form. For example, a chemical reaction occurs between two elements, the resulting products will have the same mass as the reactants.
The law of constant composition proposes that the composition of a matter will remain the same under any condition. For example, you have carbon dioxide. Carbon dioxide contains one atom of carbon and two atoms of oxygen. Even if you provide an unlimited supply of carbon, the carbon dioxide formula will remain the same. We can make more carbon dioxide by supplying more oxygen but the overall composition will always remain the same.
The above laws were used by sir John Dalton to come up with an atomic model. Although, sir John Dalton didn't perform any experiment or saw an atom because, at that time, he didn't have the right tools to prove the existence of an atom. His theory was acceptable in some ranges such as in defining the states of matter, however, many things were wrong with this theory.
J.J Thomson’s Plum-Pudding Model
Soon after the discovery of an electron, it was known that Dalton's theory about atoms was incorrect. In 1897 - 1898, a scientist, whose same is sir Joseph John Thomson (a.k.a J.J Thomson) provided a new atomic model which was acceptable at that time.
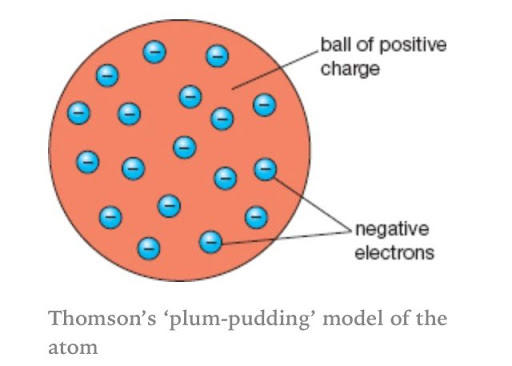
When electrons were discovered, sir J.J Thomson purposed a new model which is known as Plum-Pudding Model today. At that time, the nucleus was not discovered, therefore, sir J.J Thomson proposed that an atom contains small spheres whose radius was about 10-10 m in diameter. According to this theory, the atom contains two types of particles, positively charged particles and negatively charged particles (also known as electrons). The positive charge particles are spread uniformly and they are viewed as pudding. While the electrons are the plums that are distributed in shells. Since atoms are not charged particles, therefore, sir J.J Thomson suggested that the positively charged particles exert a force on the electrons. The direction of the force is towards the centre of the electron. The force of attraction cancels out each other hence becoming a neutral particle.
Alpha Particle Scattering Experiment
In 1911, a new concept of the atom was introduced by sir Ernest Rutherford who performed an experiment that lead to this new theory. This was the first time someone performed an experiment and provided a theory on an experimental basis. The team of sir Rutherford did an experiment using a thin gold foil. The thickness of that gold foil was 21 x 10-7 m. They placed the foil in the centre of a rotatable detector. The detector was made up of zinc sulfide and a microscope. Radioactive material was also used to bombard alpha particles on the gold foil.
When the alpha particle beam, of 5.5 MeV, was hit on the foil, the particles are scattered and this was studied by brief flashes on the screen. Team of sir Rutherford studied that most of the alpha particles crossed through the foil. This means that most of the alpha particles didn't suffer any kind of collisions. Approximately, 0.14% of the incident alpha particles were scattered by more than 1o. However, approximately 1 out of 8000 particles, the angle of deflection was even more than 90o.
Sir Rutherford concluded that alpha particles were positively charged. For them to repel back, the substance needs to be positively charged as well. At the centre, the deflection was more than 90o which showed that the mass of an atom is concentrated in the centre unlike in the previous model. Furthermore, the repelling could only occur if both particles are of the same charge. Since the deflection was noted, it concluded that the nucleus of an atom consists of protons.
The particles that were scattered by more than 1o were the particles that were near the centre of the atom. Due to the same charges, they repel and that causes the angle deflection. Last but not least, most of the particles were received as they were launched meaning most of the atom was empty. This theory lead to the discovery of the nucleus and sir Rutherford concluded that the size of the nucleus is around 10-15 and 10-14 m.

Bohr Model
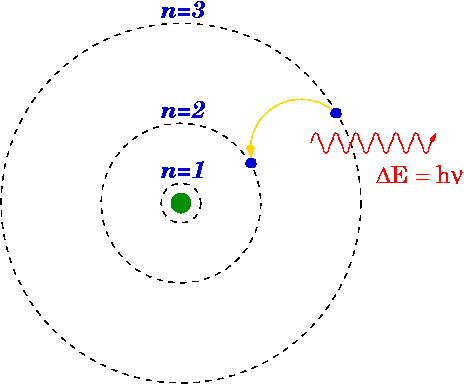
The Bohr Model was an updated form of the nuclear model. In 1913, a physicist, Niels Bohr, stated that the motion of electrons in the Rutherford model was unstable. According to the electromagnetic theory, if a charged particle moves on a curve path, it emits radiation which will cause electrons to lose energy resulting in the collision with the nucleus. However, this doesn't happen and that is what Bohr realized and came up with a new modified version of the Rutherford model. He proposed that the electrons rotate in orbits that are fixed in size and contains fixed energy. The energy of an electron is dependent on the size of the orbit. Electrons will lose or gain energy if they shift from one orbit to another.
James Chadwick Experiment
In 1932, physicist James Chadwick came up with an experiment that lead to the discovery of neutrons in a nucleus. He performed an experiment that lead to the discovery of neutrons. James Chadwick used polonium as an alpha radiation source and fired it at the beryllium sheet. This resulted in the production of uncharged, penetrating radiation. This radiation was made incident on paraffin wax. This resulted in neutrons sticking to the paraffin wax. Protons passed through the paraffin wax and were detected by an ionization chamber.
Sir James Chadwick studied the range of protons that were liberated as well as the interaction between the uncharge radiation and the atoms. Therefore, he concluded that the radiation contained some uncharge particles having the same mass as proton which were later called neutrons.













Was very educative and help. Gave me a broader understanding of the topic