Chapters
In this article, we will discuss how to convert a balanced chemical equation to an ionic equation. But before proceeding to discuss the procedure and related examples, first, let us see what is a balanced chemical equation.

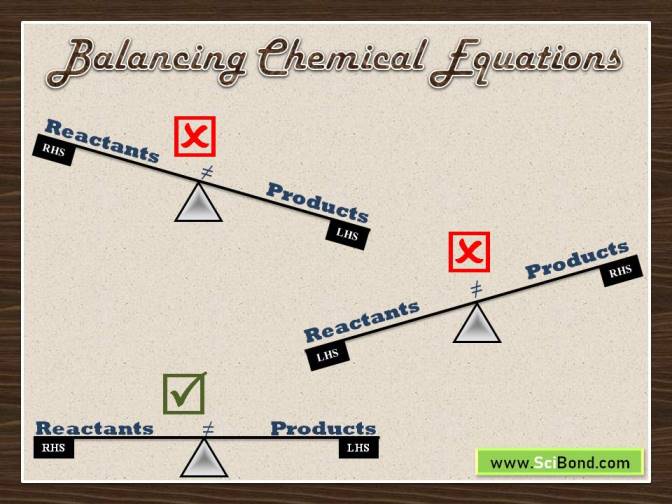
What is a Balanced Chemical Equation?
In chemical equations, the chemical symbols of each reactant and product are present. To balance an equation, there should be the same number of atoms of each element on either side of the equation. This practice is in accordance with the Law of Conservation of Mass.
Best Approach to Balance a Chemical Equation Quickly
All chemical equations should be balanced which implies that the number of atoms of each element must be the same on both sides of the equation. One of the best techniques for balancing equations quickly is to practice a lot of examples. Change the coefficients in front of the formulae and check the results on the other side of the equation. Through trial and error, you will gradually master this art. As the last step of the process, balance the elements that appear on their own.
Ionic Equations
An ionic equation refers to a chemical equation in which the electrolytes in an aqueous solution are represented as dissociated ions.
The need to write a balanced ionic equation
The ionic compounds dissociate into their ions in the aqueous solutions. It implies that they dissociate into the component ions they are made up of. Take an example of hydrochloric acid and potassium hydroxide. Both of these compounds dissociate as follows:


It is critical to recognize common ionic compounds and their constituent ions which include:
- Acids, for example, hydrochloric acid HCl and sulphuric acid

- Group I and Group II hydroxides, for instance, sodium hydroxide
- Soluble salts which include potassium sulphate and sodium chloride
Writing a Balanced Ionic Equation Worked Example
Suppose you are asked to write an ionic equation for the reaction of aqueous chlorine and aqueous potassium iodide. How will you write it?
Well, to do so, you need to follow the simple steps below:
Step 1: Write down the full balanced equation of the chemical reaction. In this example, the balanced chemical equation is:
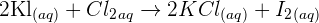
Step 2: In this step, you need to identify the ionic substances and write down the ions separately like this:

Step 3: In this step, you need to rewrite the equation by eliminating the ions which appear on both sides of the equation. The ions that appear on both sides of the equation are referred to as the spectator ions. In this example, the spectator ions are potassium ( ) ions. Hence, after eliminating these ions the new equation will be:
) ions. Hence, after eliminating these ions the new equation will be:
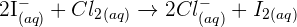
Half Equations
A half equation shows what happens to one of the reactants involved in a chemical reaction. In other words, we can say that the half reaction shows what happens when atoms or ions gain or lose electrons. The attributes of half equations are listed below:
- Electrons are represented as
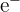
- There should be the same number of atoms of each element on both sides of the equation
- There should be the same total charge on each side of the equation (generally zero)
Half equations where positive ions gain electrons
The examples of half equations for some reactions where positive ions gain electrons are given below:
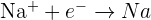
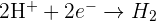

Half equations where negative ions lose electrons
The examples of half equations for some reactions where negative ions lose electrons are given below:

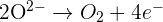
Solved Example
Take an example of the reaction that takes place between sodium and chlorine:
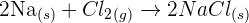
We can divide this reaction into two half equations which needs electrons to balance them. The first reaction is sodium which involves sodium that loses an electron:

The second half reaction is of chlorine in which the chlorine molecule gains two electrons:
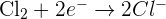
Remember that it is not necessary for half equations to have the same coefficients as the overall equations. However, it is essential to balance them in the atoms and charges.
Rules for Writing the Balanced Chemical Equation
We already know that to write the balanced ionic equation, we need to separate each compound into its component ions. Remember that it does not apply to liquids such as water and solids that are products. Any ions appearing on both sides of the equation, i.e. the spectator ions are cancelled.
Do you know that there are some rules that can help you write balanced chemical equations more easily? We have explained these rules below which will be quite helpful for you.
Rule 1: Neutralization Reactions
The neutralization reactions, i.e. the acid alkali reactions produce salt and water. We can write ionic equation as:

Remember that this rule applies to all kinds of acids and alkalis. In other words, it is applicable to monobasic acids like HCl or 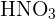 , or dibasic acids like
, or dibasic acids like  . Similarly, alkali can be sodium hydroxide NaOH or calcium hydroxide
. Similarly, alkali can be sodium hydroxide NaOH or calcium hydroxide 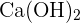 .
.
Rule 2: Precipitation Reactions
Mixing two solutions together results in the formation of a solid precipitate. These reactions are referred to as precipitation reactions. For these reactions, you should write those two ions only which make the precipitate. Consider an example of a reaction in which hydrochloric acid reacts with silver nitrate. The products of this reaction are silver chloride and nitric acid. Silver chloride is a solid and a precipitate.

Rule 3: Solid, liquid (water), and gases do not ionize
Remember that the solid, liquid (water), and gases do not ionize. However, if the reactant is a solid, one should write the chemical equation first. Write the ions in the next line, and the common ions that appear on both sides of the equation should be cancelled.
For example, solid magnesium reacts with hydrochloric acid (HCl) to form magnesium chloride and hydrogen gas. The chemical equation will be:
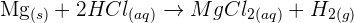
The ionic equation will be:
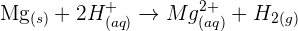
The reaction of solid magnesium carbonate with HCl produces magnesium chloride 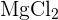 , carbon dioxide
, carbon dioxide  , and water
, and water  .
.
The chemical equation will be:
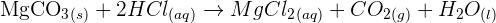
The ionic equation is given below:













Was very educative and help. Gave me a broader understanding of the topic